Seagull BioSolutions, a startup working on new biological technologies, is being funded by the Department of Science and Technology (DST), to undertake the development of Active Virosome (AV)-Vaccine and Immunodiagnostic kits for COVID-19 emergency.
Active Virosome Technology (AVT) developed by Seagull Bio is useful for the production of vaccines & immunotherapeutic agents. The AVT platform is useful for producing novel, non-hazardous & economical Active Virosome agents expressing desired antigens from the target pathogen. These will be used to develop a novel vaccine for the prevention of COVID-19 infection and also immunodiagnostic ELISA kits for COVID-19.
“Accurate diagnostics, breaking the chain of transmission, therapy and preventive measures including safe and effective vaccines are the foundational pillars of addressing the challenges of COVID-19. Of these, developing vaccines has the longest timeline and so it is essential to fast track that activity NOW,” said Professor Ashutosh Sharma, Secretary, DST.
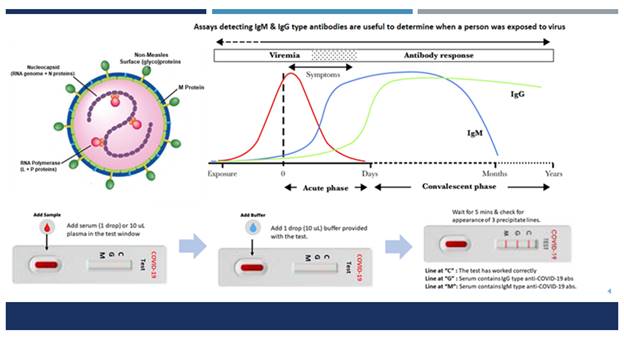
Polymerase chain reaction (PCR)-based diagnostic kits which are currently available in India are rapid & enable detection of active COVID 19 infection but cannot identify asymptomatic infections or those people who were exposed to or infected with COVID-19 in the past and did not suffer from the disease or have recovered from COVID-19 disease and may still be spreading the virus. In contrast, Immunodiagnostic kits help in detection of antibodies to COVID – which can identify these infections also. Therefore, SBPL has initiated efforts to produce Immunodiagnostic kits for COVID-19. These tests will enable healthcare researchers to monitor the spread of COVID-19 more accurately.
Seagull BioSolutions based out of Entrepreneurship Development Center (Venture Center), Pune, and supported under Seed Support System of the Technology Development Board (TDB), DST is producing two types of Active Virosome (AV) agents. SBPL will produce two types of AV agents – one expressing S protein of COVID-19 (AV-S) and another one expressing structural proteins of COVID-19 (AV-SPs). SBPL is currently amplifying the synthesis of both these agents upto 10 mg levels so that their immunogenicity can be tested. This test will first be performed in wild type mice to ascertain the ability of AV-S & AV-SPs to induce anti-COVID-19 neutralizing antibodies & cellular immune response(s).
Once this is proven, they will undertake efficacy studies in ACE-2R+ mice, which are used as a model for SARS disease. In parallel, a bioprocess for production of AV- agent will be established & the AV-agent produced in large scale about 100,000 vaccine doses. Detailed toxicity, safety & pharmacokinetic study will be performed in ACE2R+ mice and another small animal species or Monkeys, and then the AV-vaccine agent will be prepared for Phase I clinical trials. The company anticipates that the unique features of AVs will enable starting Phase I trials by the end of 18-20 months.
In parallel to this Vaccine project, SBPL will also use the Active Virosomes expressing S protein of COVID-19 as an antigen for developing immunodiagnostic kits. IgM captures ELISA kits, IgG type antibody detecting ELISA kits, and a Lateral flow (LFA) immunodiagnostic test will be produced. LFA tests will empower individual citizens of India to test themselves easily & ensure that they remain disease-free.
SBPL expects the Immunodiagnostic kits to be ready for field trials by the end of Aug 2020 and approved by the end of 10-11 months. On the other hand, the AV vaccine is expected to take a longer time. However, given the emergency situation, SBPL aims to complete the proof of concept in 80 days & complete preclinical development & start Phase I trial by the end of 18-20 months.